From 2021 to 2024 I’ve been quite fortunate to have gotten my first experiences into the MedTech world as the Business Unit Director and later CEO of a spinoff called Extra Horizon. People who know me, know that I thrive on learning the patterns across different markets. I’ve worked in data center infrastructure technology, chemical engineering analytics, maritime software, IoT platforms, enterprise blockchain, etc. Finding what’s unique to a specific domain can only be seen when you are alien to the domain itself and when you have seen enough different ones. (reading tip; the rise of the multi-domain experts).
So here are just a couple of the things I learned. Hopefully, people within the domain will find comfort in it, and people new to the market will find some early warnings 😉
The people
One of the very first things I learned in the health space in general is that the vast majority of people, from engineers to office managers, are in this domain because they care. Because they truly want to create an impact on the society through their job. In many many other markets* an engineer is an engineer and they’d easily change domains if a better opportunity comes along. Let alone office managers, to stay with the same examples. Not here! This was one of the most heartwarming experiences that has left a mark on me. THANK YOU ALL!
the founders
But here we already come to one of the most prominent differences between healthcare and ANY other market; healthcare founders are NOT entrepreneurs! They are first and foremost healthcare practitioners and researchers. They are people who live and breathe healthcare outcome for the patients, not building scalable or sustainable businesses. They are also first-time entrepreneurs. Totally different from most other domains where you usually find a 50/50 split between first-timers and serial entrepreneurs. These founders have absolutely no idea what Lean Startup means, Product Market Fit, build or buy development decisions, or what the true meanings of an MVP and a pivot contain. How and more importantly when to grow a team. And I can keep going on for a while here.
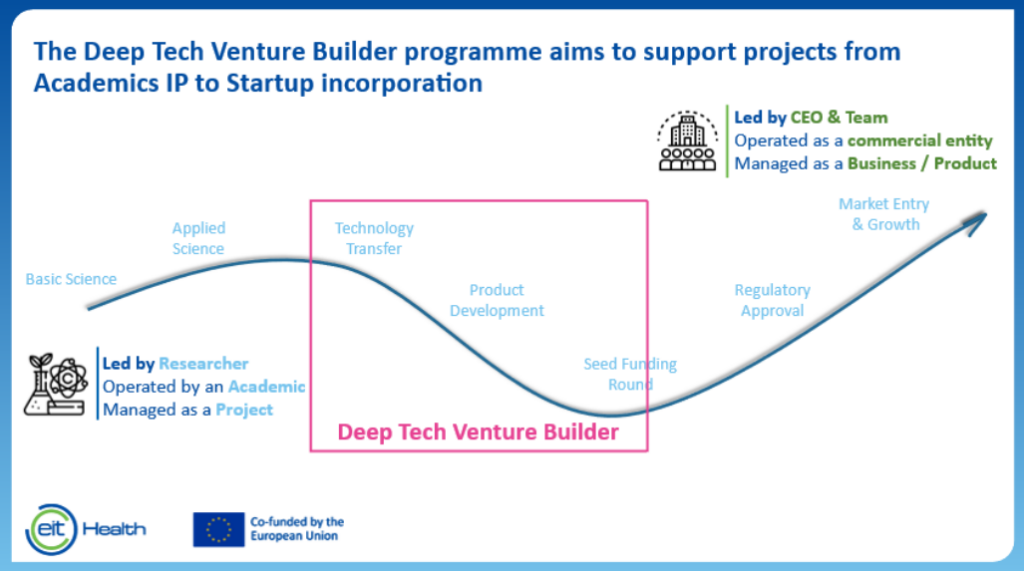
Quite recently this has also been recognized by EIT Health and in 2025 we will see the first cohort of a Deep Tech Venture Building program where the main goal is to transform deep tech academic research projects into ventures. BRAVO! More of this, please. I see a big role here for the Technology Transfer Offices (TTO’s) at all the universities in the future. These are in fact the closest entities that can make such support scalable. In my opinion, TTO’s should focus even more on boutique venture building rather than mainly IP transfer.
*caveat: other markets that I have worked in before within the technology space.
The process
the regulatory process
Many other people have much more to say about this than me as I am by far not a regulatory specialist. But I have seen what the impact of the regulatory processes is on product and company development in comparison to other domains. In short; the regulatory process of the healthcare space is by its very nature a mandatory waterfall principle. All other markets have already gained much more innovation progress by working in more agile* ways.
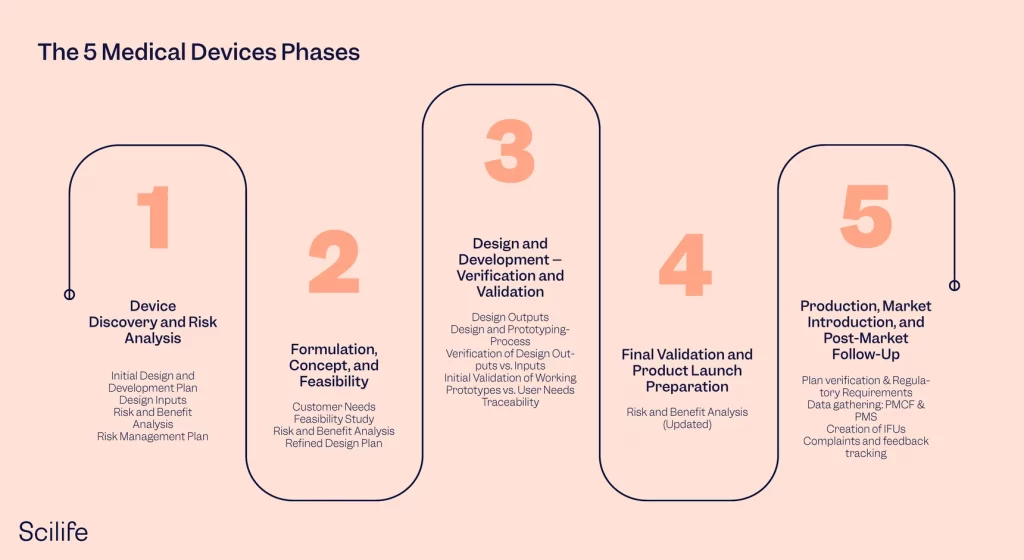
Just look up the phase-gated design validation and verification processes that are inherent to FDA (21 CFR part 820) and ISO13485 (IEC 62304) regulations. They are built on the principles of hardware device manufacturing with embedded software. For years now, medical software innovators have been struggling to fit their processes into these ancient artifacts. Yes, I do know that there are improvements on the way, but the overall mental models the people in this industry work on are still the same. MedTech is about two decades behind on modern innovation techniques and we are collectively paying the price for it!
*agile: mind you that I purposefully never use Agile with a capital letter. IYKYK
the startup process
Not only is the development process stage-gated like we’d normally think of in product versioning, but the entire business is stage-gated! Technically, that verification and validation process starts with the ideation of the product and at the end a product launch (after which it enters a post-market surveillance phase). This entire process can easily take from 10 to 15 years!!! and includes multiple clinical studies each costing millions. All of it before you can technically start testing out your “normal” product-market fit activities.
I get it, I truly do! I understand why these processes exist, historically coming from drug development. When it comes to creating products that have an active impact on the human body, there is zero margin for error (we all hope). This does come at a high cost, however: solutions that can be built today, can only benefit patients in the far future. This does not have to be the case for MedTech, especially in software, and we should embrace this much, much more!
timing
One of the consequences of having first-time entrepreneurs combined with this stage-gated venture building is that long-term company strategy is rarely a primary focus. These people are not inherently trained to look 5 years down the road, trying to anticipate important progress bottlenecks. They are focussed on the problem of today. Every major milestone in these ventures is funded separately and it is only after finalizing said milestone that the next one is prepared. Another consequence of this is that those research-entrepreneurs somewhere in their heart already know they will never be the CEO who has to grow the company. They know that at the end of the day, at some point, someone else will solve the hard venture problems after a major milestone has been reached. I have seen first-hand how this has cost a lot of time and money that would never have played out the same way in any other market.
The money
Upstream
There is still only one primary path to success in the medical market these days: getting into a reimbursement system. 99% of the startups have to focus on that. There are very few alternatives. And after that, if you are very, very lucky, at some point Big Pharma will buy you out.
However, the healthcare reimbursement systems today are still too focused on reactive healthcare versus preventative/proactive healthcare where a lot of technological innovations could play. I have seen a lot of great ideas that could benefit the health of so many people but will never hit the market because of a lack of reimbursement paths. One of the reasons I see is that preventative healthcare creates communal (socialist-leaning) gains, whereas reactive healthcare creates individualized/corporate (capitalist-leaning) gains. We need to find much better ways to validate the monetary value of preventative healthcare towards the institutions that pay for it.
Book reading tip: Upstream, how to solve problems before they happen
Pharma
I think pharma can play a big role here if they would be willing. Pharma today still pays for the majority of clinical trials and as mentioned before, of later-stage acquisitions. That is … if your solution is focused on increasing diagnostics for example. Finding more patients for their already existing drugs is something pharma is very much interested in today. However, I have seen very little evidence that pharma can and wants to play a role in MedTech in general as a new market segment for the future.
Historically, medical devices have been deployed mostly in hospital environments where device manufacturers have a monopoly. But these days, MedTech is going into home care and remote care, with much larger distribution challenges. Challenges that Pharma knows best!